*New Breakthrough Medicine* for Preventing Type 1 Diabetes
What if there was a prescription medication for patients with early-stage Type 1 Diabetes that had the potential to provide months to years without the need for insulin injections and prevention of other complications of Type 1 Diabetes?
Well, now there is with Tzield!
What is Tzield?
Tzield (Teplizumab) is a new FDA-approved medication that works by delaying the onset of Stage 3 Type 1 Diabetes. In Stage 3 Type 1 Diabetes, the beta cells in the pancreas are impaired and unable to make enough insulin, resulting in high blood sugar (hyperglycemia). Tzield acts by deactivating the immune system cells in the body that attack the beta cells of the pancreas. By using Tzield, you can prolong early-stage Type 1 Diabetes and give yourself additional time to prepare for the onset of Stage 3 Type 1 Diabetes.
Who is eligible for Tzield?
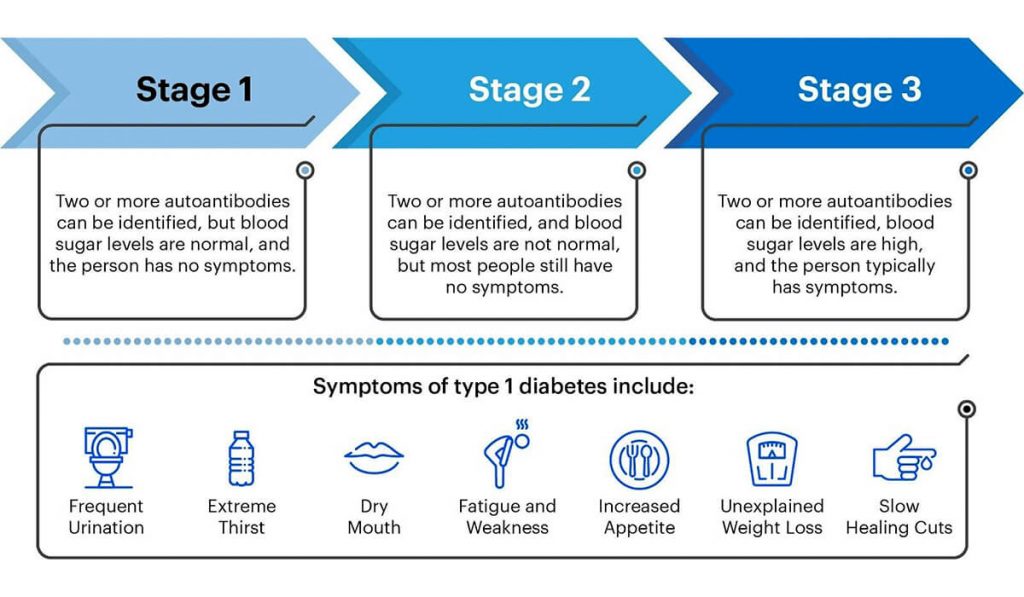
Tzield is not approved for everyone with Type 1 Diabetes. This prescription medication is specifically for adults and children of 8 years of age and older who have Stage 2 Type 1 Diabetes. If you are unsure whether or not you have Stage 2 Type 1 Diabetes, talk to your doctor about completing a blood test that looks for diabetes-related proteins called autoantibodies and abnormal blood sugars. T1 Detect also has a lot of information about detection as well. Patients with a family history of Type 1 Diabetes should reach out to their doctor about getting screened.
Safety Information and Side Effects
📌 Some common side effects of Tzield include:
- Rash
- Decreased white blood cell counts
- Headache
📌 Some serious potential side effects of Tzield include:
- Cytokine Release Syndrome
- Signs and symptoms include:
- Fever
- Nausea
- Fatigue
- Headache
- Muscle and joint pain
- Increased liver enzymes in your blood
- Signs and symptoms include:
Summary Tzield (Teplizumab) is a new FDA-approved medication for people with Stage 2 Type 1 Diabetes and is the first of its kind. This prescription medication is used for delaying the onset of Stage 3 Type 1 Diabetes. Tzield gives patients more time to prepare for Stage 3 Type 1 Diabetes control and continue living without the daily difficulties that arise with Stage 3 Type 1 Diabetes.






